American Diabetes Association Professional Practice Committee; 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes—2022. Diabetes Care 1 January 2022; 45 (Supplement_1): S46–S59. https://doi.org/10.2337/dc22-S004
Download citation file:
toolbar searchThe American Diabetes Association (ADA) “Standards of Medical Care in Diabetes” includes the ADA’s current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee (https://doi.org/10.2337/dc22-SPPC), are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA’s clinical practice recommendations, please refer to the Standards of Care Introduction (https://doi.org/10.2337/dc22-SINT). Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC.
A successful medical evaluation depends on beneficial interactions between the patient and the care team. The Chronic Care Model (1–3) (see Section 1, “Improving Care and Promoting Health in Populations,” https://doi.org/10.2337/dc22-S001) is a patient-centered approach to care that requires a close working relationship between the patient and clinicians involved in treatment planning. People with diabetes should receive health care from a coordinated interdisciplinary team that may include but is not limited to diabetes care and education specialists, primary care and subspecialty clinicians, nurses, dietitians, exercise specialists, pharmacists, dentists, podiatrists, and mental health professionals. Individuals with diabetes must assume an active role in their care. Based on patient preferences, the patient, family or support people, and health care team together formulate the management plan, which includes lifestyle management (see Section 5, “Facilitating Behavior Change and Well-being to Improve Health Outcomes,” https://doi.org/10.2337/dc22-S005).
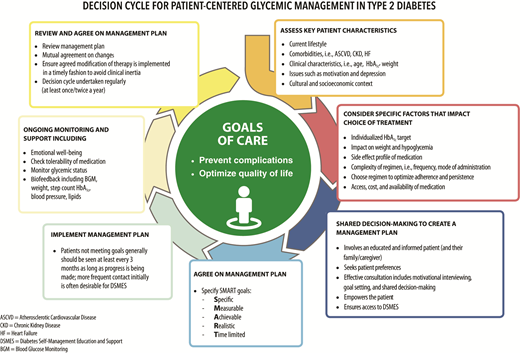
The goals of treatment for diabetes are to prevent or delay complications and optimize quality of life (Fig. 4.1). Treatment goals and plans should be created with patients based on their individual preferences, values, and goals. This individualized management plan should take into account the patient’s age, cognitive abilities, school/work schedule and conditions, health beliefs, support systems, eating patterns, physical activity, social situation, financial concerns, cultural factors, literacy and numeracy (mathematical literacy), diabetes history (duration, complications, current use of medications), comorbidities, disabilities, health priorities, other medical conditions, preferences for care, and life expectancy. Various strategies and techniques should be used to support patients’ self-management efforts, including providing education on problem-solving skills for all aspects of diabetes management.

Decision cycle for patient-centered glycemic management in type 2 diabetes. Adapted from Davies et al. (104).

Decision cycle for patient-centered glycemic management in type 2 diabetes. Adapted from Davies et al. (104).
Provider communication with patients and families should acknowledge that multiple factors impact glycemic management but also emphasize that collaboratively developed treatment plans and a healthy lifestyle can significantly improve disease outcomes and well-being (4–7). Thus, the goal of provider-patient communication is to establish a collaborative relationship and to assess and address self-management barriers without blaming patients for “noncompliance” or “nonadherence” when the outcomes of self-management are not optimal (8). The familiar terms “noncompliance” and “nonadherence” denote a passive, obedient role for a person with diabetes in “following doctor’s orders” that is at odds with the active role people with diabetes take in directing the day-to-day decision-making, planning, monitoring, evaluation, and problem-solving involved in diabetes self-management. Using a nonjudgmental approach that normalizes periodic lapses in self-management may help minimize patients’ resistance to reporting problems with self-management. Empathizing and using active listening techniques, such as open-ended questions, reflective statements, and summarizing what the patient said, can help facilitate communication. Patients’ perceptions about their own ability, or self-efficacy, to self-manage diabetes constitute one important psychosocial factor related to improved diabetes self-management and treatment outcomes in diabetes (9–11) and should be a target of ongoing assessment, patient education, and treatment planning.
The comprehensive medical evaluation includes the initial and follow-up evaluations, assessment of complications, psychosocial assessment, management of comorbid conditions, and engagement of the patient throughout the process. While a comprehensive list is provided in Table 4.1, in clinical practice the provider may need to prioritize the components of the medical evaluation given the available resources and time. The goal is to provide the health care team information so it can optimally support a patient. In addition to the medical history, physical examination, and laboratory tests, providers should assess diabetes self-management behaviors, nutrition, social determinants of health, and psychosocial health (see Section 5, “Facilitating Behavior Change and Well-being to Improve Health Outcomes,” https://doi.org/10.2337/dc22-S005) and give guidance on routine immunizations. The assessment of sleep pattern and duration should be considered; a meta-analysis found that poor sleep quality, short sleep, and long sleep were associated with higher A1C in people with type 2 diabetes (13). Interval follow-up visits should occur at least every 3–6 months individualized to the patient, and then at least annually.
Lifestyle management and psychosocial care are the cornerstones of diabetes management. Patients should be referred for diabetes self-management education and support, medical nutrition therapy, and assessment of psychosocial/emotional health concerns if indicated. Patients should receive recommended preventive care services (e.g., immunizations, cancer screening, etc.); smoking cessation counseling; and ophthalmological, dental, and podiatric referrals, as needed.
The assessment of risk of acute and chronic diabetes complications and treatment planning are key components of initial and follow-up visits (Table 4.2). The risk of atherosclerotic cardiovascular disease and heart failure (see Section 10, “Cardiovascular Disease and Risk Management,” https://doi.org/10.2337/dc22-S010), chronic kidney disease staging (see Section 11, “Chronic Kidney Disease and Risk Management,” https://doi.org/10.2337/dc22-S011), presence of retinopathy (see Section 12, “Retinopathy, Neuropathy, and Foot Care,” https://doi.org/10.2337/dc22-S012), and risk of treatment-associated hypoglycemia (Table 4.3) should be used to individualize targets for glycemia (see Section 6, “Glycemic Targets,” https://doi.org/10.2337/dc22-S006), blood pressure, and lipids and to select specific glucose-lowering medication (see Section 9, “Pharmacologic Approaches to Glycemic Treatment,” https://doi.org/10.2337/dc22-S009), antihypertension medication, and statin treatment intensity.
Assessment and treatment plan*
| Assessing risk of diabetes complications |
| • ASCVD and heart failure history |
| • ASCVD risk factors and 10-year ASCVD risk assessment |
| • Staging of chronic kidney disease (see Table 11.1) |
| • Hypoglycemia risk (see Table 4.3) |
| • Assessment for retinopathy |
| • Assessment for neuropathy |
| Goal setting |
| • Set A1C/blood glucose/time in range target |
| • If hypertension is present, establish blood pressure target |
| • Diabetes self-management goals |
| Therapeutic treatment plans |
| • Lifestyle management |
| • Pharmacologic therapy: glucose lowering |
| • Pharmacologic therapy: cardiovascular and renal disease risk factors |
| • Use of glucose monitoring and insulin delivery devices |
| • Referral to diabetes education and medical specialists (as needed) |
| Assessing risk of diabetes complications |
| • ASCVD and heart failure history |
| • ASCVD risk factors and 10-year ASCVD risk assessment |
| • Staging of chronic kidney disease (see Table 11.1) |
| • Hypoglycemia risk (see Table 4.3) |
| • Assessment for retinopathy |
| • Assessment for neuropathy |
| Goal setting |
| • Set A1C/blood glucose/time in range target |
| • If hypertension is present, establish blood pressure target |
| • Diabetes self-management goals |
| Therapeutic treatment plans |
| • Lifestyle management |
| • Pharmacologic therapy: glucose lowering |
| • Pharmacologic therapy: cardiovascular and renal disease risk factors |
| • Use of glucose monitoring and insulin delivery devices |
| • Referral to diabetes education and medical specialists (as needed) |
ASCVD, atherosclerotic cardiovascular disease.
Assessment and treatment planning are essential components of initial and all follow-up visits.
Assessment of hypoglycemia risk
| Factors that increase risk of treatment-associated hypoglycemia |
| • Use of insulin or insulin secretagogues (i.e., sulfonylureas, meglitinides) |
| • Impaired kidney or hepatic function |
| • Longer duration of diabetes |
| • Frailty and older age |
| • Cognitive impairment |
| • Impaired counterregulatory response, hypoglycemia unawareness |
| • Physical or intellectual disability that may impair behavioral response to hypoglycemia |
| • Alcohol use |
| • Polypharmacy (especially ACE inhibitors, angiotensin receptor blockers, nonselective β-blockers) |
| • History of severe hypoglycemic event |
| In addition to individual risk factors, consider use of comprehensive risk prediction models (105). |
| Factors that increase risk of treatment-associated hypoglycemia |
| • Use of insulin or insulin secretagogues (i.e., sulfonylureas, meglitinides) |
| • Impaired kidney or hepatic function |
| • Longer duration of diabetes |
| • Frailty and older age |
| • Cognitive impairment |
| • Impaired counterregulatory response, hypoglycemia unawareness |
| • Physical or intellectual disability that may impair behavioral response to hypoglycemia |
| • Alcohol use |
| • Polypharmacy (especially ACE inhibitors, angiotensin receptor blockers, nonselective β-blockers) |
| • History of severe hypoglycemic event |
| In addition to individual risk factors, consider use of comprehensive risk prediction models (105). |
Additional referrals should be arranged as necessary (Table 4.4). Clinicians should ensure that individuals with diabetes are appropriately screened for complications and comorbidities. Discussing and implementing an approach to glycemic control with the patient is a part, not the sole goal, of the patient encounter.
Referrals for initial care management
| • Eye care professional for annual dilated eye exam |
| • Family planning for women of reproductive age |
| • Registered dietitian nutritionist for medical nutrition therapy |
| • Diabetes self-management education and support |
| • Dentist for comprehensive dental and periodontal examination |
| • Mental health professional, if indicated |
| • Audiology, if indicated |
| • Social worker/community resources, if indicated |
| • Eye care professional for annual dilated eye exam |
| • Family planning for women of reproductive age |
| • Registered dietitian nutritionist for medical nutrition therapy |
| • Diabetes self-management education and support |
| • Dentist for comprehensive dental and periodontal examination |
| • Mental health professional, if indicated |
| • Audiology, if indicated |
| • Social worker/community resources, if indicated |
The importance of routine vaccinations for people living with diabetes has been elevated by the coronavirus disease 2019 (COVID-19) pandemic. Preventing avoidable infections not only directly prevents morbidity but also reduces hospitalizations, which may additionally reduce risk of acquiring infections such as COVID-19. Children and adults with diabetes should receive vaccinations according to age-appropriate recommendations (14,15). The Centers for Disease Control and Prevention (CDC) provides vaccination schedules specifically for children, adolescents, and adults with diabetes (see www.cdc.gov/vaccines/). The CDC Advisory Committee on Immunization Practices (ACIP) makes recommendations based on its own review and rating of the evidence, provided in Table 4.5 for selected vaccinations. The ACIP evidence review has evolved over time with the adoption of Grading of Recommendations Assessment, Development and Evaluation (GRADE) in 2010 and then the Evidence to Decision or Evidence to Recommendation frameworks in 2018 (16). Here we discuss the particular importance of specific vaccines.
Highly recommended immunizations for adult patients with diabetes (Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention)
| Vaccination . | Age-group recommendations . | Frequency . | GRADE evidence type* . | Reference . |
|---|---|---|---|---|
| Hepatitis B | Two- or three-dose series | 2 | Centers for Disease Control and Prevention, Use of Hepatitis B Vaccination for Adults With Diabetes Mellitus: Recommendations of the Advisory Committee on Immunization Practices (ACIP) (111) | |
| Human papilloma virus (HPV) | ≤26 years of age; 27–45 years of age may also be vaccinated against HPV after a discussion with health care provider | Three doses over 6 months | 2 for females, 3 for males | Meites et al., Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices (112) |
| Influenza | All patients; advised not to receive live attenuated influenza vaccine | Annual | – | Demicheli et al., Vaccines for Preventing Influenza in the Elderly (113) |
| Pneumonia (PPSV23 [Pneumovax]) | 19–64 years of age, vaccinate with Pneumovax | One dose | 2 | Centers for Disease Control and Prevention, Updated Recommendations for Prevention of Invasive Pneumococcal Disease Among Adults Using the 23-Valent Pneumococcal Polysaccaride Vaccine (PPSV23) (114) |
| ≥65 years of age, obtain second dose of Pneumovax, at least 5 years from prior Pneumovax vaccine | One dose; if PCV13 has been given, then give PPSV23 ≥1 year after PCV13 and ≥5 years after any PPSV23 at age | 2 | Falkenhorst et al., Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) Against Pneumococcal Disease in the Elderly: Systematic Review and Meta-analysis (115) | |
| Pneumonia (PCV13 [Prevnar]) | Adults ≥19 of age, with an immunocompromising condition (e.g., chronic renal failure), cochlear implant, or cerebrospinal fluid leak | One dose | 3 | Matanock et al., Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged ≥65 Years: Updated Recommendations of the Advisory Committee on Immunization Practices (21) |
| 19–64 years of age, immunocompetent, no recommendation | None | |||
| ≥65 years of age, immunocompetent, have shared decision-making discussion with health care provider | One dose | |||
| Tetanus, diphtheria, pertussis (TDAP) | All adults; pregnant women should have an extra dose | Booster every 10 years | 2 for effectiveness, 3 for safety | Havers et al., Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccines: Updated Recommendations of the Advisory Committee on Immunization Practices—United States, 2019 (116) |
| Zoster | ≥50 years of age | Two-dose Shingrix, even if previously vaccinated | 1 | Dooling et al., Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines (117) |
| Vaccination . | Age-group recommendations . | Frequency . | GRADE evidence type* . | Reference . |
|---|---|---|---|---|
| Hepatitis B | Two- or three-dose series | 2 | Centers for Disease Control and Prevention, Use of Hepatitis B Vaccination for Adults With Diabetes Mellitus: Recommendations of the Advisory Committee on Immunization Practices (ACIP) (111) | |
| Human papilloma virus (HPV) | ≤26 years of age; 27–45 years of age may also be vaccinated against HPV after a discussion with health care provider | Three doses over 6 months | 2 for females, 3 for males | Meites et al., Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices (112) |
| Influenza | All patients; advised not to receive live attenuated influenza vaccine | Annual | – | Demicheli et al., Vaccines for Preventing Influenza in the Elderly (113) |
| Pneumonia (PPSV23 [Pneumovax]) | 19–64 years of age, vaccinate with Pneumovax | One dose | 2 | Centers for Disease Control and Prevention, Updated Recommendations for Prevention of Invasive Pneumococcal Disease Among Adults Using the 23-Valent Pneumococcal Polysaccaride Vaccine (PPSV23) (114) |
| ≥65 years of age, obtain second dose of Pneumovax, at least 5 years from prior Pneumovax vaccine | One dose; if PCV13 has been given, then give PPSV23 ≥1 year after PCV13 and ≥5 years after any PPSV23 at age | 2 | Falkenhorst et al., Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) Against Pneumococcal Disease in the Elderly: Systematic Review and Meta-analysis (115) | |
| Pneumonia (PCV13 [Prevnar]) | Adults ≥19 of age, with an immunocompromising condition (e.g., chronic renal failure), cochlear implant, or cerebrospinal fluid leak | One dose | 3 | Matanock et al., Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged ≥65 Years: Updated Recommendations of the Advisory Committee on Immunization Practices (21) |
| 19–64 years of age, immunocompetent, no recommendation | None | |||
| ≥65 years of age, immunocompetent, have shared decision-making discussion with health care provider | One dose | |||
| Tetanus, diphtheria, pertussis (TDAP) | All adults; pregnant women should have an extra dose | Booster every 10 years | 2 for effectiveness, 3 for safety | Havers et al., Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccines: Updated Recommendations of the Advisory Committee on Immunization Practices—United States, 2019 (116) |
| Zoster | ≥50 years of age | Two-dose Shingrix, even if previously vaccinated | 1 | Dooling et al., Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines (117) |
GRADE, Grading of Recommendations Assessment, Development and Evaluation; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
Evidence type: 1 = randomized controlled trials (RCTs), or overwhelming evidence from observational studies; 2 = RCTs with important limitations, or exceptionally strong evidence from observational studies; 3 = observational studies, or RCTs with notable limitations; and 4 = clinical experience and observations, observational studies with important limitations, or RCTs with several major limitations. For a comprehensive list, refer to the Centers for Disease Control and Prevention at www.cdc.gov/vaccines/.
Influenza is a common, preventable infectious disease associated with high mortality and morbidity in vulnerable populations, including youth, older adults, and people with chronic diseases. Influenza vaccination in people with diabetes has been found to significantly reduce influenza and diabetes-related hospital admissions (17). In patients with diabetes and cardiovascular disease, influenza vaccine has been associated with lower risk of all-cause mortality, cardiovascular mortality, and cardiovascular events (18). Given the benefits of the annual influenza vaccination, it is recommended for all individuals ≥6 months of age who do not have a contraindication. Influenza vaccination is critically important in the next year as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza viruses will both be active in the U.S. during the 2021–2022 season (19). The live attenuated influenza vaccine, which is delivered by nasal spray, is an option for patients who are age 2 years through age 49 years and who are not pregnant, but patients with chronic conditions such as diabetes are cautioned against taking the live attenuated influenza vaccine and are instead recommended to receive the inactive or recombinant influenza vaccination. For individuals ≥65 years of age, there may be additional benefit from the high-dose quadrivalent inactivated influenza vaccine (19).
Like influenza, pneumococcal pneumonia is a common, preventable disease. People with diabetes are at increased risk for the bacteremic form of pneumococcal infection and have been reported to have a high risk of nosocomial bacteremia, with a mortality rate as high as 50% (20). There are two vaccination types, the 23-valent pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13), with distinct schedules for children and adults.
All children are recommended to receive a four-dose series of PCV13 by 15 months of age. For children with diabetes who have incomplete series by ages 2–5 years, the CDC recommends a catch-up schedule to ensure that these children have four doses. Children with diabetes between 6–18 years of age are also advised to receive one dose of PPSV23, preferably after receipt of PCV13.
For adults with diabetes, one dose of PPSV23 is recommended between the ages of 19 and 64 years and another dose at ≥65 years of age. The PCV13 is no longer routinely recommended for patients over 65 years of age because of the declining rates of pneumonia attributable to these strains (21). Older patients should have a shared decision-making discussion with their provider to determine individualized risks and benefits. PCV13 is recommended for patients with immunocompromising conditions such as asplenia, advanced kidney disease, cochlear implants, or cerebrospinal fluid leaks (22). Some older patients residing in assisted living facilities may also consider PCV13. If the PCV13 is to be administered, it should be given prior to the next dose of PPSV23.
Compared with the general population, people with type 1 or type 2 diabetes have higher rates of hepatitis B. This may be due to contact with infected blood or through improper equipment use (glucose monitoring devices or infected needles). Because of the higher likelihood of transmission, hepatitis B vaccine is recommended for adults with diabetes aged
As of August 2021, the COVID-19 vaccines are recommended for all adults and some children, including people with diabetes, under full approval of the U.S. Food and Drug Administration. The three options in the U.S. are the mRNA vaccines from Pfizer-BioNTech and Moderna and the recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector vaccine from Janssen. Pfizer-BioNTech vaccine is recommended for people aged 12 years and older, with a grade 1 evidence rating for the prevention of symptomatic COVID-19 (23,24). It is given as a two-shot series 21 days apart. Moderna vaccine is recommended for people aged 18 years and older, with a grade 1 evidence rating for prevention of symptomatic COVID-19 (23). It is given as a two-shot series 28 days apart. Janssen vaccine is also recommended for people aged 18 years and older, with a grade 2 evidence rating (25). Unlike the mRNA vaccines, only one shot is required. Evidence regarding the efficacy of mixing vaccines is still emerging. Booster vaccine recommendations are also evolving, with the CDC just recently recommending the Pfizer-BioNTech booster for older adults and those with underlying conditions such as diabetes. The COVID-19 vaccine will likely become a routine part of the annual preventive schedule for people with diabetes.
Besides assessing diabetes-related complications, clinicians and their patients need to be aware of common comorbidities that affect people with diabetes and that may complicate management (26–30). Diabetes comorbidities are conditions that affect people with diabetes more often than age-matched people without diabetes. This section discusses many of the common comorbidities observed in patients with diabetes but is not necessarily inclusive of all the conditions that have been reported.
People with type 1 diabetes are at increased risk for other autoimmune diseases, with thyroid disease, celiac disease, and pernicious anemia (vitamin B12 deficiency) being among the most common (31). Other associated conditions include autoimmune hepatitis, primary adrenal insufficiency (Addison disease), collagen vascular diseases, and myasthenia gravis (32–35). Type 1 diabetes may also occur with other autoimmune diseases in the context of specific genetic disorders or polyglandular autoimmune syndromes (36). Given the high prevalence, nonspecific symptoms, and insidious onset of primary hypothyroidism, routine screening for thyroid dysfunction is recommended for all patients with type 1 diabetes. Screening for celiac disease should be considered in adult patients with suggestive symptoms (e.g., diarrhea, malabsorption, abdominal pain) or signs (e.g., osteoporosis, vitamin deficiencies, iron deficiency anemia) (37,38). Measurement of vitamin B12 levels should be considered for patients with type 1 diabetes and peripheral neuropathy or unexplained anemia.
Diabetes is associated with increased risk of cancers of the liver, pancreas, endometrium, colon/rectum, breast, and bladder (39). The association may result from shared risk factors between type 2 diabetes and cancer (older age, obesity, and physical inactivity) but may also be due to diabetes-related factors (40), such as underlying disease physiology or diabetes treatments, although evidence for these links is scarce. Patients with diabetes should be encouraged to undergo recommended age- and sex-appropriate cancer screenings and to reduce their modifiable cancer risk factors (obesity, physical inactivity, and smoking). New onset of atypical diabetes (lean body habitus, negative family history) in a middle-aged or older patient may precede the diagnosis of pancreatic adenocarcinoma (41). However, in the absence of other symptoms (e.g., weight loss, abdominal pain), routine screening of all such patients is not currently recommended.
Diabetes is associated with a significantly increased risk and rate of cognitive decline and an increased risk of dementia (42,43). A recent meta-analysis of prospective observational studies in people with diabetes showed 73% increased risk of all types of dementia, 56% increased risk of Alzheimer dementia, and 127% increased risk of vascular dementia compared with individuals without diabetes (44). The reverse is also true: people with Alzheimer dementia are more likely to develop diabetes than people without Alzheimer dementia. In a 15-year prospective study of community-dwelling people >60 years of age, the presence of diabetes at baseline significantly increased the age- and sex-adjusted incidence of all-cause dementia, Alzheimer dementia, and vascular dementia compared with rates in those with normal glucose tolerance (45). See Section 13, “Older Adults” (https://doi.org/10.2337/dc22-S013), for a more detailed discussion regarding screening for cognitive impairment.
In those with type 2 diabetes, the degree and duration of hyperglycemia are related to dementia. More rapid cognitive decline is associated with both increased A1C and longer duration of diabetes (44). The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study found that each 1% higher A1C level was associated with lower cognitive function in individuals with type 2 diabetes (46). However, the ACCORD study found no difference in cognitive outcomes in participants randomly assigned to intensive and standard glycemic control, supporting the recommendation that intensive glucose control should not be advised for the improvement of cognitive function in individuals with type 2 diabetes (47).
In type 2 diabetes, severe hypoglycemia is associated with reduced cognitive function, and those with poor cognitive function have more severe hypoglycemia. In a long-term study of older patients with type 2 diabetes, individuals with one or more recorded episodes of severe hypoglycemia had a stepwise increase in risk of dementia (48). Likewise, the ACCORD trial found that as cognitive function decreased, the risk of severe hypoglycemia increased (49). Tailoring glycemic therapy may help to prevent hypoglycemia in individuals with cognitive dysfunction. See Section 13, “Older Adults” (https://doi.org/10.2337/dc22-S013), for more detailed discussion of hypoglycemia in older patients with type 1 and type 2 diabetes.
In one study, adherence to the Mediterranean diet correlated with improved cognitive function (50). However, a recent Cochrane review found insufficient evidence to recommend any specific dietary change for the prevention or treatment of cognitive dysfunction (51).
A systematic review has reported that data do not support an adverse effect of statins on cognition (52). The U.S. Food and Drug Administration postmarketing surveillance databases have also revealed a low reporting rate for cognitive-related adverse events, including cognitive dysfunction or dementia, with statin therapy, similar to rates seen with other commonly prescribed cardiovascular medications (52). Therefore, fear of cognitive decline should not be a barrier to statin use in individuals with diabetes and a high risk for cardiovascular disease.
Diabetes is associated with the development of nonalcoholic fatty liver disease (NAFLD), including its more severe manifestations of nonalcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis, and hepatocellular carcinoma (53). Elevations of hepatic transaminase concentrations are associated with higher BMI, waist circumference, and triglyceride levels and lower HDL cholesterol levels. Noninvasive tests, such as elastography or fibrosis biomarkers, may be used to assess risk of fibrosis, but referral to a liver specialist and liver biopsy may be required for definitive diagnosis (54). Interventions that improve metabolic abnormalities in patients with diabetes (weight loss, glycemic control, and treatment with specific drugs for hyperglycemia or dyslipidemia) are also beneficial for fatty liver disease (55,56). Pioglitazone, vitamin E treatment, liraglutide, and semaglutide treatment of biopsy-proven NASH have each been shown to improve liver histology, but effects on longer-term clinical outcomes are not known (57–59). Treatment with other glucagon-like peptide 1 receptor agonists and with sodium–glucose cotransporter 2 inhibitors has shown promise in preliminary studies, although benefits may be mediated, at least in part, by weight loss (59–61).
The American Gastroenterological Association convened an international conference, including representatives of the ADA, to review and discuss published literature on burden, screening, risk stratification, diagnosis, and management of individuals with NAFLD, including NASH (62). Please see the special report “Preparing for the NASH Epidemic: A Call to Action” for full details (62). Significant gaps were identified, including gaps in knowledge in who to screen and how to diagnose and treat patients at high risk for NASH. In patients with suspected NAFLD, diagnosis consists of evaluating patients for alternative or coexisting causes of liver disease through history and laboratory testing. In patients with NAFLD/NASH, risk stratification with noninvasive fibrosis scores was suggested. Table 4.6, reproduced from the special report, summarizes the management recommendations for patients with NAFLD and NASH, and Table 4.7 presents the summary of published NAFLD guidelines included in the the report (62). Further research and interdisciplinary consensus are required to fully define screening, referral, and diagnostic pathways.
Management of patients with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis
| Variable . | Lifestyle intervention a . | Liver-directed pharmacotherapy . | Diabetes care (in individuals with diabetes) . | Cardiovascular risk reduction . |
|---|---|---|---|---|
| NAFLD | Yes | No | Standard of care | Yes |
| NASH with fibrosis stage 0 or 1 (F0, F1) | Yes | No | Standard of care | Yes |
| NASH with fibrosis stage 2 or 3 (F2, F3) | Yes | Yes | Pioglitazone, GLP-1 receptor agonists b | Yes |
| NASH cirrhosis (F4) | Yes | Yes | Individualize c | Yes |
| Variable . | Lifestyle intervention a . | Liver-directed pharmacotherapy . | Diabetes care (in individuals with diabetes) . | Cardiovascular risk reduction . |
|---|---|---|---|---|
| NAFLD | Yes | No | Standard of care | Yes |
| NASH with fibrosis stage 0 or 1 (F0, F1) | Yes | No | Standard of care | Yes |
| NASH with fibrosis stage 2 or 3 (F2, F3) | Yes | Yes | Pioglitazone, GLP-1 receptor agonists b | Yes |
| NASH cirrhosis (F4) | Yes | Yes | Individualize c | Yes |
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
All patients require regular physical activity and healthy diet and to avoid excess alcohol intake; weight loss recommended.
Among glucagon-like peptide 1 (GLP-1) receptor agonists, semaglutide has the best evidence of benefit in patients with NASH and fibrosis.
Evidence for efficacy of pharmacotherapy in patients with NASH cirrhosis is very limited and should be individualized and used with caution. Adapted from “Preparing for the NASH Epidemic: A Call to Action” (62).
Summary of published nonalcoholic fatty liver disease guidelines
| Organization . | Year . | First-line diagnosis test . | When to refer to hepatologist . | Noninvasive tests . |
|---|---|---|---|---|
| American Association for the Study of Liver Diseases (AASLD) | 2018 | Not clear in the guideline Routine screening for NAFLD in high-risk groups is not recommended | Not clear in the guideline | Diagnosis for NASH: liver biopsy Assessment for fibrosis: NFS or FIB-4 |
| American Gastroenterological Association (AGA) | 2012 | Routine screening for NAFLD is not recommended | Not clear in the guideline | Metabolic syndrome can be used to target patients for liver biopsy |
| European Association for the Study of the Liver (EASL) | 2016 | Ultrasound + liver enzymes for patients with risk factors | Refer patients with abnormal liver enzymes or medium-/high-risk fibrosis markers to specialist | Diagnosis for NASH: liver biopsy Assessment for fibrosis: NFS or FIB-4 |
| World Gastroenterology Organization (WGO) | 2012 | Ultrasound + liver enzymes for patients with risk factors | Not clear in the guideline | Diagnosis for NASH: liver biopsy |
| National Institute for Health Care and Excellence (NICE) | 2016 | Ultrasound + liver enzymes for patients with risk factors But routine liver function blood tests are not sensitive, and ultrasound is not cost-effective | Refer adults with advanced liver fibrosis to a hepatologist Refer children with suspected NAFLD to a pediatric specialist in hepatology | Assessment for advanced fibrosis: enhanced liver fibrosis (every 2–3 years) |
| Organization . | Year . | First-line diagnosis test . | When to refer to hepatologist . | Noninvasive tests . |
|---|---|---|---|---|
| American Association for the Study of Liver Diseases (AASLD) | 2018 | Not clear in the guideline Routine screening for NAFLD in high-risk groups is not recommended | Not clear in the guideline | Diagnosis for NASH: liver biopsy Assessment for fibrosis: NFS or FIB-4 |
| American Gastroenterological Association (AGA) | 2012 | Routine screening for NAFLD is not recommended | Not clear in the guideline | Metabolic syndrome can be used to target patients for liver biopsy |
| European Association for the Study of the Liver (EASL) | 2016 | Ultrasound + liver enzymes for patients with risk factors | Refer patients with abnormal liver enzymes or medium-/high-risk fibrosis markers to specialist | Diagnosis for NASH: liver biopsy Assessment for fibrosis: NFS or FIB-4 |
| World Gastroenterology Organization (WGO) | 2012 | Ultrasound + liver enzymes for patients with risk factors | Not clear in the guideline | Diagnosis for NASH: liver biopsy |
| National Institute for Health Care and Excellence (NICE) | 2016 | Ultrasound + liver enzymes for patients with risk factors But routine liver function blood tests are not sensitive, and ultrasound is not cost-effective | Refer adults with advanced liver fibrosis to a hepatologist Refer children with suspected NAFLD to a pediatric specialist in hepatology | Assessment for advanced fibrosis: enhanced liver fibrosis (every 2–3 years) |
FIB-4, Fibrosis-4 Index; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NFS, NAFLD fibrosis score. Adapted from “Preparing for the NASH Epidemic: A Call to Action” (62).
Infection with hepatitis C virus (HCV) is associated with a higher prevalence of type 2 diabetes, which is present in up to one-third of individuals with chronic HCV infection. HCV may impair glucose metabolism by several mechanisms, including directly via viral proteins and indirectly by altering proinflammatory cytokine levels (63). The use of newer direct-acting antiviral drugs produces a sustained virological response (cure) in nearly all cases and has been reported to improve glucose metabolism in individuals with diabetes (64). A meta-analysis of mostly observational studies found a mean reduction in A1C levels of 0.45% (95% CI −0.60 to −0.30) and reduced requirement for glucose-lowering medication use following successful eradication of HCV infection (65).
Diabetes is linked to diseases of the exocrine pancreas such as pancreatitis, which may disrupt the global architecture or physiology of the pancreas, often resulting in both exocrine and endocrine dysfunction. Up to half of patients with diabetes may have some degree of impaired exocrine pancreas function (66). People with diabetes are at an approximately twofold higher risk of developing acute pancreatitis (67).
Conversely, prediabetes and/or diabetes has been found to develop in approximately one-third of patients after an episode of acute pancreatitis (68); thus, the relationship is likely bidirectional. Postpancreatitis diabetes may include either new-onset disease or previously unrecognized diabetes (69). Studies of patients treated with incretin-based therapies for diabetes have also reported that pancreatitis may occur more frequently with these medications, but results have been mixed and causality has not been established (70–72).
Islet autotransplantation should be considered for patients requiring total pancreatectomy for medically refractory chronic pancreatitis to prevent postsurgical diabetes. Approximately one-third of patients undergoing total pancreatectomy with islet autotransplantation are insulin free 1 year postoperatively, and observational studies from different centers have demonstrated islet graft function up to a decade after the surgery in some patients (73–77). Both patient and disease factors should be carefully considered when deciding the indications and timing of this surgery. Surgeries should be performed in skilled facilities that have demonstrated expertise in islet autotransplantation.
Age-specific hip fracture risk is significantly increased in both people with type 1 diabetes (relative risk 6.3) and those with type 2 diabetes (relative risk 1.7) in both sexes (78). Type 1 diabetes is associated with osteoporosis, but in type 2 diabetes, an increased risk of hip fracture is seen despite higher bone mineral density (BMD) (79). In three large observational studies of older adults, femoral neck BMD T-score and the World Health Organization Fracture Risk Assessment Tool (FRAX) score were associated with hip and nonspine fractures. Fracture risk was higher in participants with diabetes compared with those without diabetes for a given T-score and age or for a given FRAX score (80). Providers should assess fracture history and risk factors in older patients with diabetes and recommend measurement of BMD if appropriate for the patient’s age and sex. Fracture prevention strategies for people with diabetes are the same as for the general population and may include vitamin D supplementation. For patients with type 2 diabetes with fracture risk factors, thiazolidinediones (81) and sodium–glucose cotransporter 2 inhibitors (82) should be used with caution.
Hearing impairment, both in high-frequency and low- to midfrequency ranges, is more common in people with diabetes than in those without, with stronger associations found in studies of younger people (83). Proposed pathophysiologic mechanisms include the combined contributions of hyperglycemia and oxidative stress to cochlear microangiopathy and auditory neuropathy (84). In a National Health and Nutrition Examination Survey (NHANES) analysis, hearing impairment was about twice as prevalent in people with diabetes compared with those without, after adjusting for age and other risk factors for hearing impairment (85). Low HDL cholesterol, coronary heart disease, peripheral neuropathy, and general poor health have been reported as risk factors for hearing impairment for people with diabetes, but an association of hearing loss with blood glucose levels has not been consistently observed (86). In the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort, time-weighted mean A1C was associated with increased risk of hearing impairment when tested after long-term (>20 years) follow-up (87). Impairment in smell, but not taste, has also been reported in individuals with diabetes (88).
Mean levels of testosterone are lower in men with diabetes compared with age-matched men without diabetes, but obesity is a major confounder (89,90). Testosterone replacement in men with symptomatic hypogonadism may have benefits including improved sexual function, well-being, muscle mass and strength, and bone density (91). In men with diabetes who have symptoms or signs of low testosterone (hypogonadism), a morning total testosterone level should be measured using an accurate and reliable assay (92). In men who have total testosterone levels close to the lower limit, it is reasonable to determine free testosterone concentrations either directly from equilibrium dialysis assays or by calculations that use total testosterone, sex hormone binding globulin, and albumin concentrations (92). Please see the Endocrine Society clinical practice guideline for detailed recommendations (92). Further tests (such as luteinizing hormone and follicle-stimulating hormone levels) may be needed to further evaluate the patient. Testosterone replacement in older men with hypogonadism has been associated with increased coronary artery plaque volume, with no conclusive evidence that testosterone supplementation is associated with increased cardiovascular risk in hypogonadal men (92).
Age-adjusted rates of obstructive sleep apnea, a risk factor for cardiovascular disease, are significantly higher (4- to 10-fold) with obesity, especially with central obesity (93). The prevalence of obstructive sleep apnea in the population with type 2 diabetes may be as high as 23%, and the prevalence of any sleep-disordered breathing may be as high as 58% (94,95). In participants with obesity enrolled in the Action for Health in Diabetes (Look AHEAD) trial, it exceeded 80% (96). Patients with symptoms suggestive of obstructive sleep apnea (e.g., excessive daytime sleepiness, snoring, witnessed apnea) should be considered for screening (97). Sleep apnea treatment (lifestyle modification, continuous positive airway pressure, oral appliances, and surgery) significantly improves quality of life and blood pressure control. The evidence for a treatment effect on glycemic control is mixed (98).
Periodontal disease is more severe, and may be more prevalent, in patients with diabetes than in those without and has been associated with higher A1C levels (99–101). Longitudinal studies suggest that people with periodontal disease have higher rates of incident diabetes. Current evidence suggests that periodontal disease adversely affects diabetes outcomes, although evidence for treatment benefits remains controversial (30,102). In a randomized clinical trial, intensive periodontal treatment was associated with better glycemic control (A1C 8.3% vs. 7.8% in control subjects and the intensive-treatment group, respectively) and reduction in inflammatory markers after 12 months of follow-up (103).
A complete list of members of the American Diabetes Association Professional Practice Committee can be found at https://doi.org/10.2337/dc22-SPPC.
Suggested citation: American Diabetes Association Professional Practice Committee. 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S46–S59